
Israeli Device Destroys Some Malignant Tumors
US doctors already use IceCure’s ProSense to destroy benign breast lumps. Soon they’ll be using it for malignant liver and kidney tumors.
In July 2012, ISRAEL21c wrote about a novel device from Caesarea-based IceCure Medical that was revolutionizing how American doctors remove benign breast lumps.
The device uses minimally invasive cryoablation to destroy the tumor by freezing it rather than surgically removing it.
Since then, IceCure’s ProSense system has expanded its applications dramatically.
The device currently is used in Asia, Israel and Europe to zap early-stage cancerous liver and kidney tumors. IceCure recently received US Food and Drug Administration (FDA) approval to do the same in the United States.
Just how soon ProSense will be available in US treatment centers for the two new applications is hard to say.
IceCure Medical VP of Business Development and Global Marketing Tlalit Bussi Tel Tzure tells ISRAEL21c that the insurance reimbursement codes are in place for both indications.
Now the public company is beginning to “build the right infrastructure to start our commercial phase for these indications” in America from its New Jersey branch office.
Mission: treating more patients
Meantime, IceCure’s ProSense device is being used in Europe for breast, kidney, lung and bone cancer.
And more than 300 lung cancer patients and 300 breast cancer patients have been treated with ProSense in Japan.

“In Israel we are performing a clinical trial for kidney cancer in Bnai Zion Medical Center in Haifa. We have treated about 100 patients with very promising results. We hope in the coming year to finalize this study and publish our results,” says Tel Tzure.
“We have also started registration in China and already have our first order from a leading Chinese hospital to do an independent study for breast cancer,” she adds.
IceCure is working toward gaining FDA approval for using ProSense to treat cancerous breast tumors.
“At the end of 2014, we started a landmark study in the United States using our technology to treat early diagnosed breast cancer with cryoablation instead of surgery,” says Tel Tzure.
“The trial is now in 19 centers, and at the end of 2019 we finished enrollment of 200 patients. We will follow up for five years to see if there has been no reoccurrence of the tumor at the area that was treated. Only then can we apply to the FDA for approval for the breast cancer indication. It will be a major breakthrough when that happens.”
In October last year, the American Association of Breast Surgeons adapted its guidelines based on IceCure’s clinical trial results, supporting “accelerated investigations of cryoablation technology for treatment of malignant breast tumors.”
IceCure also has received FDA approval for ear, nose and throat applications. This is not yet an active focus but could be in the future.
“The world wants to use our innovation to treat more patients. So this is our mission,” says Tel Tzure.
How ProSense works
IceCure was cofounded in 2006 by cryogenics expert Dr. Alex Levin and businessman Didier Toubia (now cofounder and CEO of cultivated meat startup Aleph Farms) in the Naiot Venture Accelerator in Yokne’am.
The cryoablation device they developed is based on a technology Levin patented in 2002.
The physician inserts a ProSense probe into the tumor – there are different ones corresponding to the size and shape of the mass – guided by ultrasound or CT imaging.
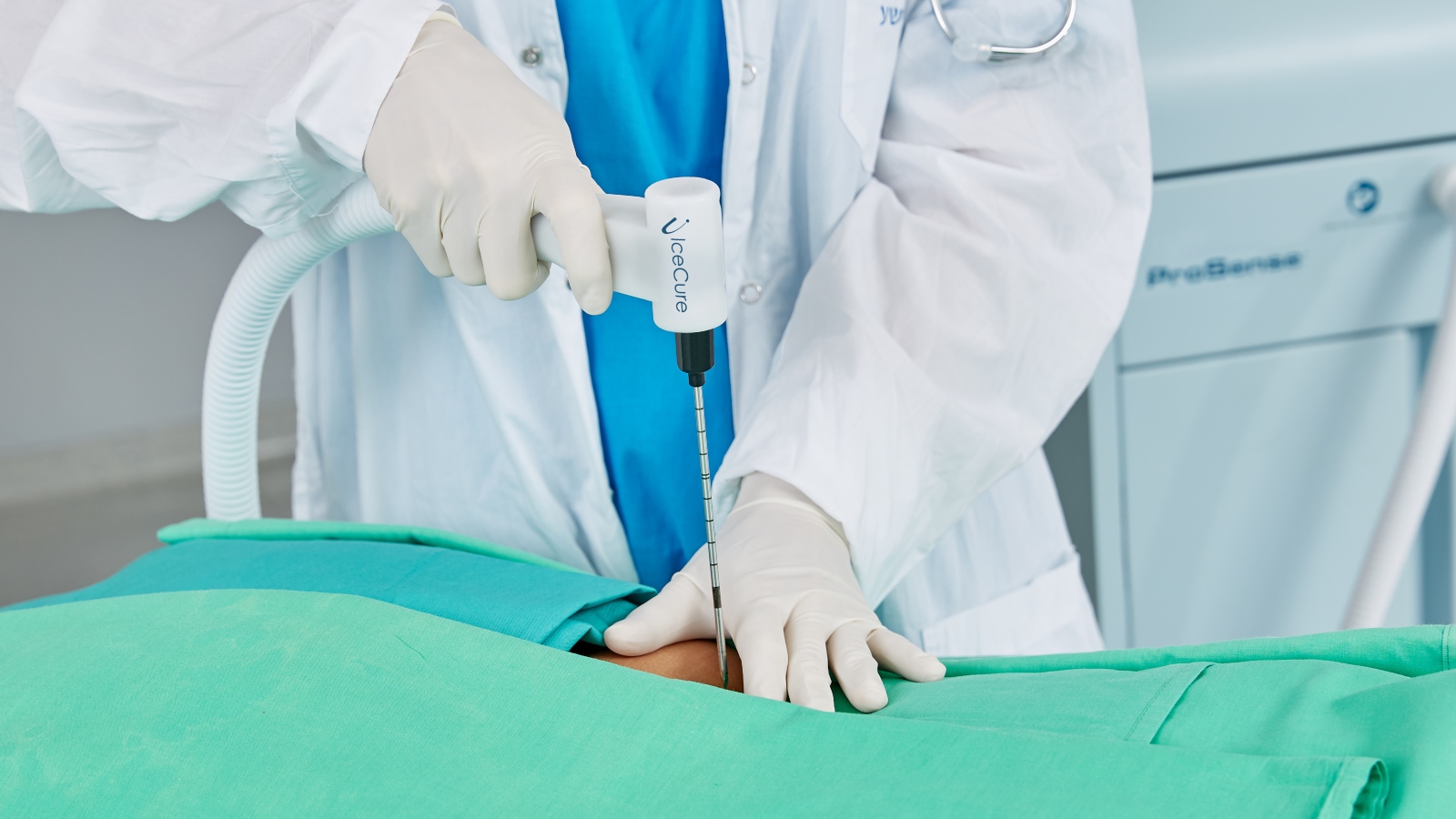
Once the needle is inside, the probe tip’s temperature is lowered with liquid nitrogen to minus 170 Celsius in less than a minute. Within an hour, the extreme cold destroys the target tissue, which dissolves out of the body naturally in a few weeks.
The procedure is done under local anesthesia no matter where the tumor is located. The surrounding healthy tissue is not damaged and there is no scarring. Afterward, the patient can go right home and resume full activity.
There are several limitations on this groundbreaking treatment: It cannot treat multiple tumors and it is only for early-stage (stage 1 or 2) tumors confined to no more than 1.5 centimeters in the breast or up to 3 centimeters in the liver, kidney or bone.
“With early screening, the number of tumors being found at this stage is growing all the time,” notes Tel Tzure.
“It is important to mention that our technology does not replace chemotherapy or radiation therapy. Depending on the patient, the care team may want to follow up with those therapies.”


